

Global Non-Invasive Prenatal Testing (NIPT) Market.
Dublin, May 22, 2024 (GLOBE NEWSWIRE) — Non-Invasive Prenatal Testing (NIPT) Market – A Global and Regional Analysis: Focus on Method, Test, Platform, End-User, Application and Region – Analysis and Forecast, 2023-2033 ” the report added ResearchAndMarkets.com’s The offer of
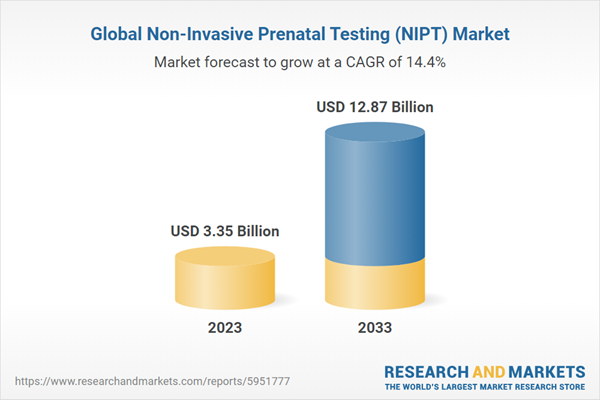
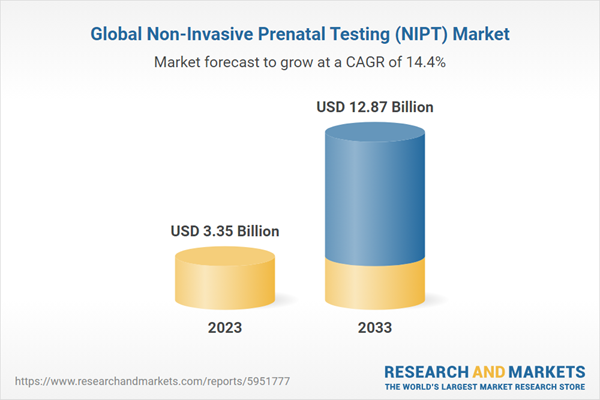
The global NIPT market was valued at USD 3,350.4 million in 2023 and is expected to reach USD 12,870.9 million by 2033, registering a CAGR of 14.41% during the forecast period 2023-2033
The base year considered for calculating the market size is 2022. The historical year analysis is done from FY2020 to FY2021, and the market size is calculated for FY2022 and projected for the period 2023-2033. The global NIPT market is primarily driven by increasing NIPT encouraging guidelines, payer reimbursement for screening procedures, continuous technology innovation, commercial potential across geographies, and growing preferences for non-invasive testing methods.
The NIPT market in the Asia-Pacific region is witnessing a significant growth of 15.43% in the forecast period, characterized by increasing number of market players and changing healthcare landscape. In 2022, Asia-Pacific accounted for a share of 19.50% of the global NIPT market.
The global NIPT market is characterized by intense competition, as established entities and emerging players compete for market share. The anticipated growth and transformation of the market brings challenges and opportunities, making it a dynamic landscape to watch in the coming years.
Impact on industry
Confluence of micro and macro trends such as increasing awareness of early detection and prevention of chromosomal abnormalities, increasing adoption of non-invasive methods and consumption of diagnostic tests are driving the market towards future growth. The industry is expected to witness technological leaps with offerings beyond chromosomal abnormalities moving from research to clinical testing, early pregnancy testing enabling faster intervention and increased affordability due to technological advances and wider insurance coverage. Increasing research and development to address current constraints and unmet needs will further drive market growth in the future.
Industry advances in NIPT research and development continually address population health trends, disease prevalence, and treatment outcomes. As a result, the impact of the NIPT market extends beyond technology integration for diagnostics, making it an integral component of global health strategies and the broader ecosystem.
Key Market Players and Competitive Overview
NIPT refers to a method of examining fetal deoxyribonucleic acid (DNA) to determine the risk of genetic abnormalities in the developing fetus. Testing is usually done by taking a blood sample from a pregnant woman and analyzing small fragments of DNA that circulate in a pregnant woman’s blood. The test helps detect chromosomal abnormalities, especially the trisomies that cause Down’s, Edward’s and Patau’s syndromes.
The global non-invasive prenatal testing market is in a growth phase and is expanding rapidly, creating opportunities for emerging players that embrace non-PCR targeted assays or sequencing methods to enable wider adoption and challenge the position of established players. big ones in the market like Natera, Illumina, LabCorp, Roche. Molecular Systems/BioReference Laboratories, and BGI Genomics.
Trisomy Detection to Dominate Global Non-Invasive Prenatal Testing Market (by Application)
The trisomy detection segment dominated the global NIPT market (by application) in FY2022.
NIPT’s high specificity, efficacy and safety (over 99% for trisomy 21), increasing access, increasing incidence of chromosomal abnormalities, and increasing maternal age are collectively driving market growth.
Hospitals to dominate global non-invasive prenatal testing market (by end user)
Larger patient database due to existing maternity and prenatal care services, specialized medical staff in public and private hospitals, easier access to genetic counselors and other specialists for pre- and post-test consultations, and potential workflow integrated with diagnostic procedures such as amniocentesis in case of positive NIPT results are driving the growth of the hospital segment.
NGS to dominate global non-invasive prenatal testing market (by platform)
The global NIPT market (by platform) was dominated by the NGS segment in FY2022. NIPT tests based on NGS are more widespread due to the difference in its affordability, high throughput and accuracy, integration with other technologies that enable efficient data interpretation and shorter turnaround times.
cfDNA to dominate global NIPT market (by method)
The global NIPT market (by method) was dominated by the cfDNA segment in FY2022. cfDNA analysis is being explored for additional applications such as determining fetal sex, identifying single-gene disorders, and even assessing growth-promoting fetal health and development.
Main attributes:
|
The report attribute |
The details |
|
number of pages |
180 |
|
Forecast period |
2023 – 2033 |
|
Estimated market value (USD) in 2023 |
3.35 billion dollars |
|
Projected market value (USD) by 2033 |
12.87 billion dollars |
|
Compound Annual Growth Rate |
14.4% |
|
Regions covered |
global |
Overview of market dynamics
Market leaders
-
High incidence of genetic disorders
-
Increasing age of the mother
-
Increasing the number of reimbursement policies towards genetic coverage
-
Increased emphasis on early detection and prevention
Trends: Assessing current and future impact
Market restrictions
-
Strict regulatory guidelines and ethical hurdles
-
Lack of awareness about NIPT
-
Limitations and considerations in non-invasive prenatal testing
Market Opportunities
Research and Development Review
The Regulatory Landscape
-
The regulatory scenario in the US
-
Regulatory Scenario in Canada
-
The regulatory scenario in the UK
-
Regulatory Scenario in Germany
-
The Regulatory Scenario in France
-
The regulatory scenario in Spain
-
Regulatory Scenario in Italy
-
Regulatory Scenario in the Netherlands
-
The regulatory scenario in China
-
Regulatory scenario in India
-
Regulatory Scenario in Australia
-
The regulatory scenario in Japan
Refund scenario
Supply chain overview
Featured companies
-
Agilent Technologies, Inc.
-
F. Hoffmann-La Roche Ltd
-
PerkinElmer, Inc.
-
Research diagnostics are incorporated
-
Illumina, Inc.
-
Myriad Genetics, Inc.
-
BGI Genomics
-
CENTOGENE NV
-
Laboratory Corporation of America Holdings
-
MedGenome Inc.
-
Annoroad Gene Technology
-
Natera, Inc.
-
Yourgene Health
-
Eurofins Scientific SE
-
Future Biosciences
For more information about this report, visit https://www.researchandmarkets.com/r/orwkg4
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world’s leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, top industries, top companies, new products and the latest trends.
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood,Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900

#Noninvasive #prenatal #testing #NIPT #markets #Opportunities #arising #growing #markets #Asia #increasing #focus #noninvasive #preimplantation #genetic #testing #niPGT
Image Source : finance.yahoo.com